CLP1 mutation causes Pontocelebellar hypoplasia Type 10
We analyzed the in vivo function of CLP1, a molecule involved in the transfer RNA (tRNA) maturation mechanism, and discovered that a genetic mutation in CLP1 is coincidentally involved in neuronal degeneration (Hanada et al. Nature 2013)
Furthermore, a family with a mutation in the human CLP1 gene was identified in Turkey, indicating that mutations in the human CLP1 gene can also cause neurodegenerative diseases in humans (Karaca et al. Cell 2014).

We demonstrated that a CLP1 homozygous missense mutation (p.R140H) caused progressive neurodegenerative disorders through newly established CLP1-R140H knock-in mouse model (Clp1RH/RH). Clp1RH/RH mice showed progressive neurological disorders, motor ataxia, and microcephaly.
Morisaki et al. BBRC 2021

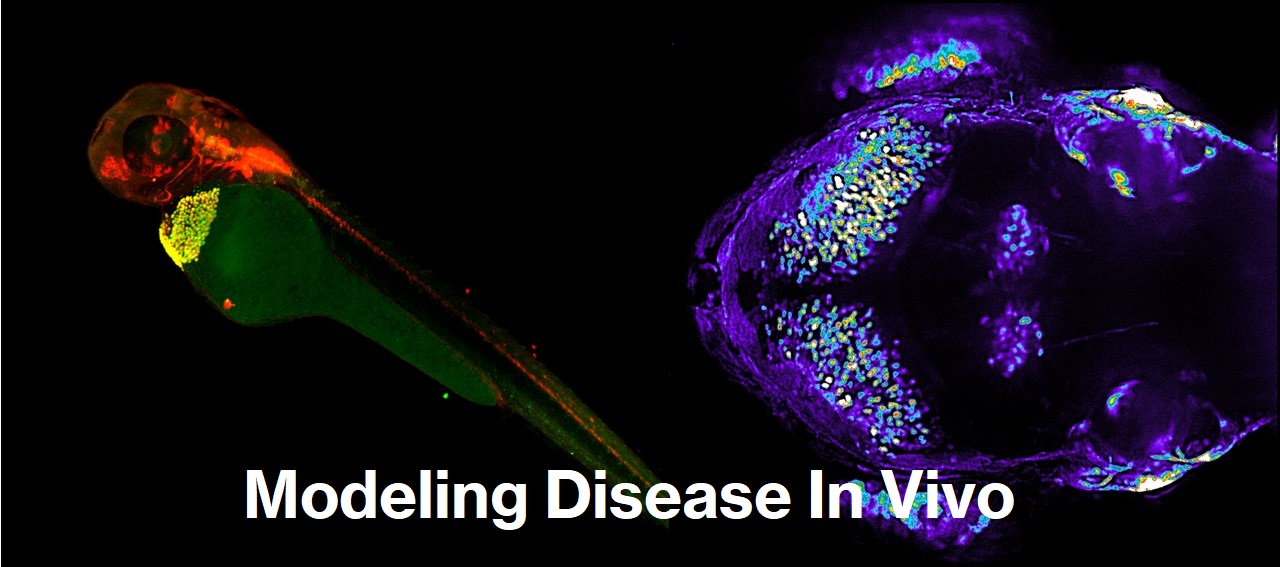
Zebrafish Model for Rare Disease Biology
Due to recent advancements in genome editing technologies, including CRISPR/Cas9, it has become possible to perform sophisticated genetic modifications across various animal species. Zebrafish, in particular, allow for relatively easy genetic modifications, enabling the rapid creation of diverse models. Additionally, due to their transparency, the developmental stages from fertilized eggs can be visualized, making it possible to analyze the onset of diseases from early embryonic stages.
Furthermore, their ability to be housed in large numbers within limited space and at low cost makes in vivo screening feasible.